A study looking at treatment of cancer of the penis that has spread to the lymph nodes (InPACT)
Cancer type:
Status:
Phase:
This study is to see what the best order of treatment is for men who have advanced penile cancer. It is for men whose penile cancer has spread to the lymph nodes in the area where your tummy (abdomen) ends and your legs begin (groin) or the area between your hip bones (pelvis).
Cancer Research UK supports this study.
More about this trial
Penis cancer usually spreads first to the lymph nodes in the groin. From there it might spread to lymph nodes deeper in the pelvis.

Surgery to remove all or part of the penis is the most common treatment for men with penile cancer that has spread to the lymph nodes in the groin.
There are 4 other treatments men might have. These are:
- surgery to remove the lymph nodes in the groin near where the cancer first appeared – this is an inguinal lymph node dissection (ILND)
- chemotherapy then ILND
- chemoradiotherapy then ILND
- surgery to remove lymph nodes deeper in the pelvis, away from where the cancer first appeared, where there is a high chance cancer cells could be- this is a prophylactic pelvic lymph node dissection (PLND)
At the moment doctors don’t know the best order to use these treatments in and so they want to find this out.
To do this they are going to compare treatments in 2 parts. These are called InPACT- neoadjuvant and InPACT- pelvis. Only men with a high risk of their cancer coming back will take part in InPACT- pelvis.
The aims of this study are to find out:
- if having surgery after chemotherapy or after chemoradiotherapy is better than just having surgery
- if having another operation to remove lymph nodes deeper into the pelvis is better than not having this operation
- more about the quality of life of men with penile cancer
Who can enter
The following bullet points list the entry conditions for this study. Talk to your doctor or the study team if you are unsure about any of these. They will be able to advise you.
You may be able to join this study if all of the following apply. You
- Have a squamous cell penile cancer
- Have a tumour of any size and have cancer in at least one lymph node in the groin area (Stage any T, N1 – 3, M0)
- Are well enough to be up and about for at least half the day (performance status 0,1 or 2)
- Have satisfactory blood test results
- Have a cancer that can be seen and measured on a scan
- Are able to have the study treatments that your doctors think are suitable for you
- Are willing to use reliable contraception during the treatment and for 6 months after if there is any chance your partner could become pregnant
- Are aged 18 or over
As well as the above to take part in the InPACT- pelvis part of the study you must have a high risk of your cancer coming back. This will be based on the results of the examination of your lymph nodes taken out in InPACT- neoadjuvant.
You cannot join this study if any of these apply. You
- Have a type of penile cancer called pure verrucous or non - squamous
- Have squamous cancer of the urethra
- Have cancer that has spread to another part of your body (Stage M1)
- Have already had chemotherapy or chemoradiotherapy for your penile cancer
- Have had another cancer, apart from squamous cell skin cancer (SCC) or basal cell carcinoma skin cancer (that was not on the penis), that needed treatment in the last 3 years
Trial design
This is an international phase 3 study. The researchers need about 200 men to take part worldwide.
There are 2 parts to the study:
- InPACT – neoadjuvant
- InPACT – pelvis
InPACT – neoadjuvant
Neoadjuvant therapy means giving cancer treatment before surgery to try and make it more successful.
Everyone has surgery to remove lymph nodes in the groin.
If your doctor thinks chemotherapy or chemoradiotherapy is not suitable for you then you just have surgery.
If your doctors decide you can safely have chemotherapy or chemoradiotherapy you enter the randomised part of the study.
The men taking part are put into 1 of 3 treatment groups by a computer.
- no treatment before surgery to remove the lymph nodes in the groin
- TIP chemotherapy then surgery to remove the lymph nodes in the groin
- chemoradiotherapy then surgery to remove lymph nodes in the groin

In certain circumstances you might only be able to be put into 2 out of the 3 groups. This is based on how suitable your doctor thinks each treatment is for you.
Or, in other circumstances, your doctor might be able to choose which group you are in. This is based on which treatment or treatments you are able to have.
The lymph nodes that are removed during your surgery are looked at carefully by a pathologist. They can see how many of the lymph nodes have cancer cells in them. Your doctor will then talk to you about the risk of your cancer spreading further.
If there is a high risk of your cancer spreading further you might have the option of taking part in the InPACT– pelvis study.
InPACT – pelvis
InPACT - pelvis is the part of the study looking at penile cancer that has spread to the pelvic lymph nodes. To take part you will have already had treatment in InPACT- neoadjuvant.
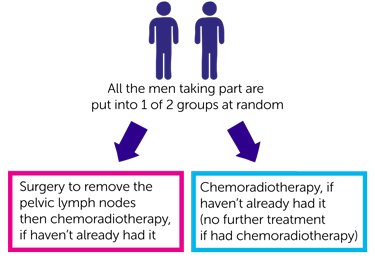
Everyone taking part is put into 1 of 2 treatment groups at random by a computer.
- surgery to remove the pelvic lymph nodes then chemoradiotherapy, if you haven’t already had it
- no operation to remove the pelvic lymph nodes but chemoradiotherapy, if you haven’t already had it (you have no further treatment if you have had chemoradiotherapy)
Neither you nor your doctor can choose which group you are in.
Samples for research
The study team will ask your permission to use a sample of tissue that was removed when you had surgery before you entered the study.
The team will use these samples to learn more about why some men develop penile cancer and respond differently to treatment. The researchers hope this knowledge could help people in the future.
You do not have to agree to give these samples for research. You can still take part in the main study.
Quality of life study
The study team ask you to complete questionnaires to find out more about any side affects you have and how you are feeling. Each questionnaire takes about 20 minutes to complete.
You fill in the first questionnaire before you start treatment. You then fill in questionnaires during and after you finish treatment:
- about every 3 months for the first year
- about every 6 months for the second year
- at 36 months (3 years)
Hospital visits
You see the doctor and have some tests before you start treatment. These tests include:
- a physical examination
- blood tests
- CT scan or MRI scan
Your doctor will talk to you about how long they expect you to be in hospital for surgery to remove the lymph nodes in your groin. Most men are in hospital for a few days and recover well at home.
If you are in the group having TIP chemotherapy you have these drugs as a drip into the vein every 3 weeks. Each 3 week period is called a cycle of treatment. You have 4 cycles of treatment. You have this as either a day patient or you may need to stay in hospital.
If you have chemoradiotherapy you go to the radiotherapy department Monday to Friday for 5 weeks. Each treatment takes about 5 minutes. You also go to the hospital one day a week to have cisplatin, which is the chemotherapy part of the treatment. You have the cisplatin as a drip into the vein. This takes about 5 hours.
If you have a second surgery to the lymph nodes in the pelvis you stay in hospital for several days. It normally takes a couple of weeks after that to recover at home.
During treatment you might have some CT or MRI scans, and after treatment you have some CT or MRI scans. Your doctor will tell you how often you have these.
During the trial, you will see the doctor about every:
- 3 months for the first 2 years
- 6 months for the next 3 years
You have a CT or an MRI scan every 6 months for the first 2 years.
Side effects
The side effects of surgery to remove your lymph nodes might include:
- fluid leaking from around the groin
- fluid collecting in the wound
- swelling of your leg, groin or genital area (lymphoedema)
- infection in the wound
The most common side effect from TIP chemotherapy is a drop in blood cells causing an increased risk of infection, bleeding problems, tiredness and breathlessness.
We have more information about the side effects of TIP.
The side effects of chemoradiotherapy can include:
- a skin reaction that can look like sunburn- your doctor or nurse will tell you how to look after your skin and can give you creams and painkillers if necessary
- swelling of your leg, groin or genital area (lymphoedema)
- a drop in blood cells causing an increased risk of infection, bleeding problems, tiredness and breathlessness
We have more information about the side effects of radiotherapy to the pelvis.
The study team will go through all the possible side effects from treatment with you before you start in the study.
Location
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Dr Steve Nicholson
Supported by
Cancer Research UK
Institute of Cancer Research (ICR)
Other information
This is Cancer Research UK trial number CRUK/13/005.
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040



