A trial looking at different treatments including carfilzomib for people with myeloma (CARDAMON)
Cancer type:
Status:
Phase:
This trial looked at carfilzomib and the timing of a  for people with myeloma.
for people with myeloma.
It was for people with myeloma who had not had treatment.
Cancer Research UK supported this trial.
This trial was open for people to join between 2015 and 2019. The team published these results in 2022.
More about this trial
Doctors treat newly diagnosed myeloma with a combination of drugs. There are stages to treatment:
induction treatment 
consolidation treatment 
- a transplant using your own stem cells
maintenance treatment 
This trial looked at:
- induction treatment
- timing of a stem cell transplant
- maintenance treatment
Carfilzomib is a  called a cancer growth blocker. It stops signals that cancer cells use to divide and grow.
called a cancer growth blocker. It stops signals that cancer cells use to divide and grow.
When the trial was done the standard induction treatment was bortezomib, cyclophosphamide and dexamethasone. Researchers thought that replacing carfilzomib for bortezomib might be better.
They also thought having more of this treatment (consolidation treatment) might be able to delay having the stem cell transplant.
The aims of this trial were to find out:
- how well carfilzomib, cyclophosphamide and dexamethasone work
- whether further treatment after induction is an acceptable option to having a stem cell transplant straight after induction treatment
- how well carfilzomib works as a maintenance treatment
Summary of results
The team found that consolidation treatment did not meet the required aims and goals to be considered as a replacement for stem cell transplant. However the results were close enough that it may be a suitable treatment for certain people.
Trial design
This was a  . There were 2 parts to the trial. 281 people were to have treatment as part of the trial.
. There were 2 parts to the trial. 281 people were to have treatment as part of the trial.
Part 1
278 people had carfilzomib, cyclophosphamide and dexamethasone as their induction treatment.
Part 2
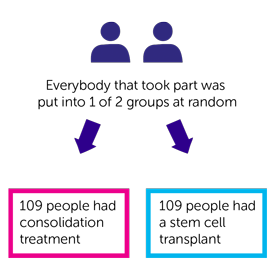
This part was  . A computer put 218 of the 278 people who were in part 1 into a treatment group. The 2 groups were:
. A computer put 218 of the 278 people who were in part 1 into a treatment group. The 2 groups were:
- 109 people had consolidation treatment
- 109 people had a stem cell transplant
After their consolidation treatment or stem cell transplant everyone was to have maintenance therapy. This was carfilzomib and dexamethasone.
The trial team looked at the results based on the intended treatment for these people. They weren’t looking at the actual treatment they had. This is an  .
.
Results
The team looked at how well the induction treatment worked. They found that 162 people (57.7%) had a very good partial response. This means there was little evidence of the myeloma in their blood after treatment.
After 2 years the team looked at how many people were alive and still free of myeloma. They found that it was:
- 68 out of every 100 people (68%) who had consolidation treatment
- 75 out of every 100 people (75%) who had a stem cell transplant
They also looked at the overall number of people who were still alive at 2 years. They found that it was:
- just under 91 out of every 100 people (90.8%) who had the consolidation treatment
- over 94 out of every 100 people (94.4%) who had the stem cell transplant
Side effects
The most common side effects people had were:
- a drop in the red blood cells and the white blood cells
- high blood pressure
- infections
Conclusion
The team say that further trials using consolidation therapy to delay having a stem cell transplant would be acceptable. Particularly for those who have little or no sign of myeloma after the induction treatment.
A French study group is running a trial for people who have little sign of their myeloma after induction treatment. It is comparing:
- having a transplant
- consolidation therapy
More detailed information
There is more information about this research in the reference below.
Please note, this article is not in plain English. It has been written for healthcare professionals and researchers.
Kwee Yong, William Wilson, Ruth M de Tute, Marquita Camilleri et al.
Lancet Haematology 2023; 10: e93–106
Where this information comes from
We have based this summary on the information in the article above. This has been reviewed by independent specialists  and published in a medical journal. We have not analysed the data ourselves. As far as we are aware, the link we list above is active and the article is free and available to view.
and published in a medical journal. We have not analysed the data ourselves. As far as we are aware, the link we list above is active and the article is free and available to view.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Professor Kwee Yong
Supported by
Amgen
Cancer Research UK
University College London (UCL)
Other information
This is Cancer Research UK trial number CRUK/13/032.
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040