A trial of megestrol acetate and letrozole for women with breast cancer (PIONEER)
Please note - this trial is no longer recruiting patients. We hope to add results when they are available.
Cancer type:
Status:
Phase:
This trial is comparing letrozole and megestrol acetate with letrozole on its own before surgery to remove breast cancer. It is for women who:
- can no longer become pregnant (
post menopausal  )
) - have breast cancer that is sensitive to the female hormone oestrogen (
oestrogen receptor positive  or ER positive)
or ER positive)
More about this trial
Women with  often have treatment with hormone therapy before surgery. This can help to slow the growth of the cancer so that you can have a smaller operation.
often have treatment with hormone therapy before surgery. This can help to slow the growth of the cancer so that you can have a smaller operation.
A common hormone therapy that women who have had their menopause have is letrozole (Femara). But doctors are looking at news ways to help women in this situation. In this trial, they are looking at megestrol acetate.
Megestrol acetate (Megace) is also a hormone therapy. It is a man made version of the hormone  . Doctors think that taking megestrol acetate with letrozole may be better at slowing the growth of the cancer, than letrozole alone.
. Doctors think that taking megestrol acetate with letrozole may be better at slowing the growth of the cancer, than letrozole alone.
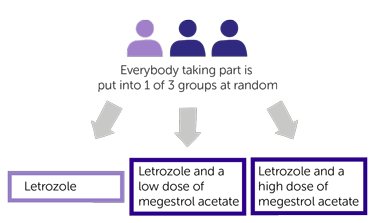
Everyone taking part in this trial has 1 of the following:
- letrozole (treatment A)
- letrozole and a low dose of megestrol acetate (treatment B)
- letrozole and a high dose of megestrol acetate (treatment C)
After finishing hormone treatment, everyone has surgery to remove the breast cancer.
The main aim of this trial is to find out if megestrol acetate and letrozole is better than letrozole alone before surgery for breast cancer.
Who can enter
The following bullet points list the entry conditions for this trial. Talk to your doctor or the trial team if you are unsure about any of these. They will be able to advise you.
Who can take part
You may be able to join this trial if you are a woman and all of the following apply:
- you have early stage breast cancer and are post menopausal
- your breast cancer is sensitive to the female hormone oestrogen (oestrogen receptor positive) and does not have receptors for a protein called
HER2  (HER2 negative)
(HER2 negative) - doctors think that surgery to remove breast cancer is the best treatment for you
- you are going to have breast cancer surgery in the next 2 to 6 weeks
- you are well enough to be up and about for at least half the day (performance status of 0, 1 or 2)
- you have satisfactory blood tests results
- you can swallow and absorb tablets
Who can’t take part
You cannot join this trial if any of these apply:
- you have breast cancer that has come back (recurrent)
- your breast cancer has spread to other parts of the body (metastatic)
- you have had
hormone replacement therapy  , an
, an aromatase inhibitor  or the drug tamoxifen in the last 6 months
or the drug tamoxifen in the last 6 months - you have had an experimental treatment in the past 4 weeks
- you are known to be sensitive to lactose, megestrol acetate or any other similar drug
- you have an intra uterine device (
IUD  or coil) that contains progesterone
or coil) that contains progesterone - you have any other serious medical condition that the trial team thinks could affect you taking part
Trial design
This is a phase 2 trial. The researchers hope that around 189 women will take part.
This trial is randomised. Women are put into 1 of the following treatment groups by computer:
- letrozole (treatment A)
- letrozole and a low dose of megestrol acetate (treatment B)
- letrozole and a high dose of megestrol acetate (treatment C)
Neither you nor your doctor are able to decide which group you are in. For every 2 women in treatment A, there are 3 women in treatment B and 3 in treatment C.
Letrozole and megestrol acetate are tablets that you take every day, once a day. You swallow them whole, with or without food.
You take letrozole, or letrozole and megestrol acetate, for up to 19 days until the morning of your operation. Then everyone has surgery to remove the breast cancer. This is the same as the standard treatment. Your doctor can tell you more about this.
Diary
The trial team will give you a diary to complete at home. They will ask you to keep a record of when you take the tablets.
You return the diary to the team at the end of the trial.
Tissue sample
You need to give a tissue sample (biopsy) before the start of treatment and during surgery.
The research team wants to:
- look for any changes that may have been caused by letrozole and megestrol acetate
- look at the way certain proteins (receptors) interact with the cancer
DNA 
Blood tests
You also have extra blood tests as part of this trial. You have them before the start of letrozole, or before the start of letrozole and megestrol acetate, and at the end of treatment.
Hospital visits
You see a doctor and have some tests before taking part. These tests include:
- physical examination
- blood tests
You go to hospital to collect the study drug and the diary. The trial team will tell you when to start taking the drugs.
You speak with the trial team after 5 and 10 days of the start of treatment. This is so they can find out how you are and what side effects you have had.
You take letrozole, or letrozole and megestrol acetate, for up to 19 days and stop it on the morning of your operation.
You then see the trial doctor 2 weeks after surgery.
Side effects
The trial team monitor you while you are having treatment. They will give you a phone number to call them if you are worried about anything.
The team thinks you are unlikely to have any side effects from taking megestrol acetate. This is because you only take them for up to 19 days. The team will tell you about all the possible side effects before you start the trial.
The most common side effects of taking megestrol acetate for a long period of time are:
- increase in appetite and weight gain
- hot flushes
- rounded face
- high blood pressure
- raised blood sugar levels
- constipation
- shortness of breath
- blood clots in the lungs or veins
We have more information about the side effects of megestrol acetate. And information about:
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Dr Richard Baird
Supported by
Anticancer Fund
Cambridge University Hospitals NHS Foundation Trust
Cancer Research UK Cambridge Centre
University of Cambridge
National Institute for Health Research
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040



